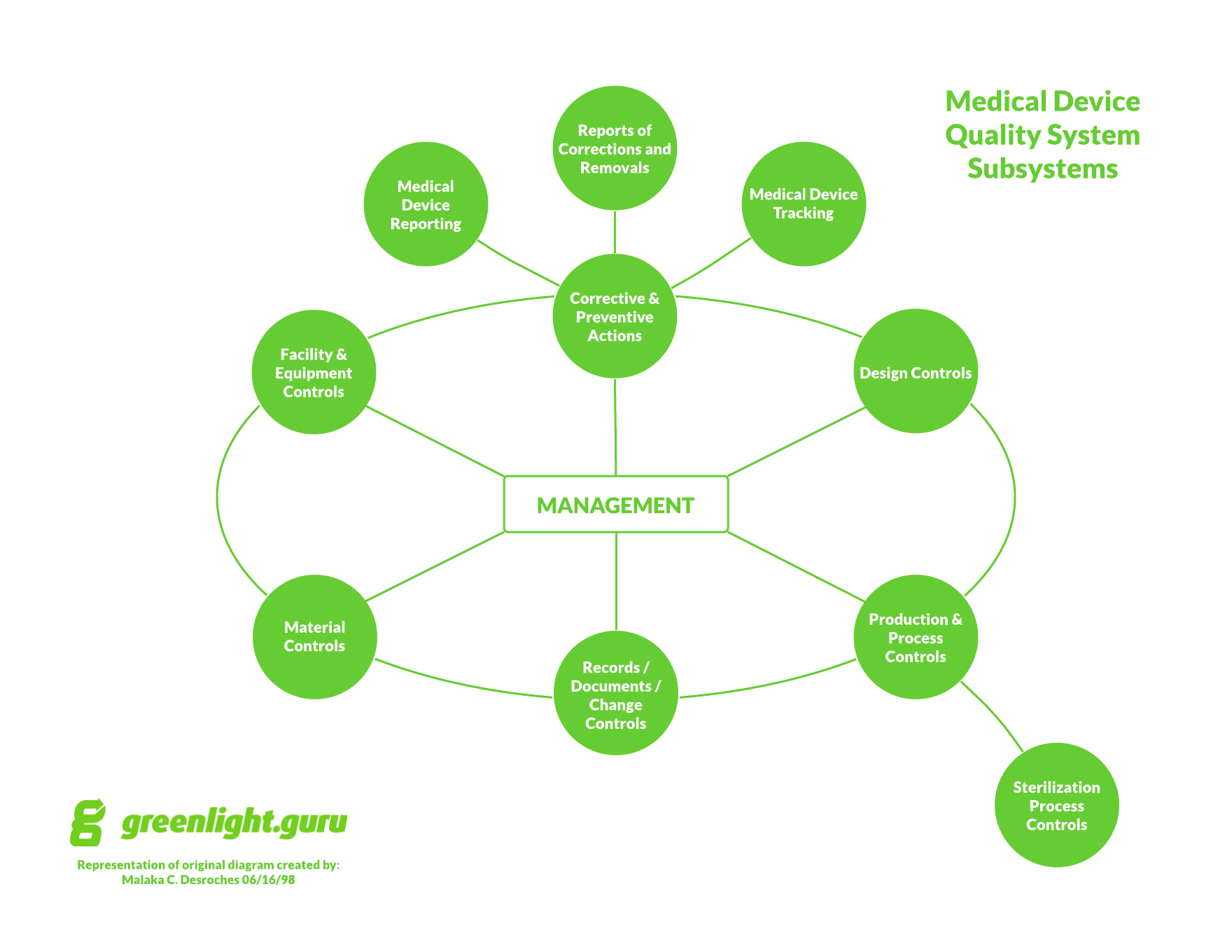
Step-by-step guide to complying with medical device QMS requirements
Medical device companies, listen up. There is zero excuse for not complying with medical device quality system regulations. Zero. Why? FDA has published and makes available ALL regulations required for medical device companies. Look them up by searching 21 CFR part 820. And for outside U.S., you can easily obtain ISO 13485 for a relatively […]

